1. The Natural greenhouse effect
1. The Natural greenhouse effect
1.2. The Greenhouse Gases (GHG)
The greenhouse gases are components of the atmosphere that can ‘absorb’ and release infrared rays (heat energy). These gases have three or more atoms in their molecules. This in contrast to the gases making up more than 99% of the atmosphere: nitrogen, oxygen and argon having only two atoms in their molecules.
Water vapor (H2O)
The most important greenhouse gas is water vapor, which at times can approach 2% in parts of the atmosphere. However, when water vapor condenses into clouds, the white upside reflects sunlight back into space, which has a negative forcing effect.
Water vapor cannot initiate radiative forcing, it can only respond to radiative forcing through feedback mechanisms. Water vapor in the air is fairly constant, and human activities have little impact on this. Since humans have little or no direct impact on the amount of water in the air, and since it is not a forcing factor, water vapor is not included in negotiations on the reduction of emissions of greenhouse gases.
With increasing temperatures there will be increasing evaporation and increased amounts of water vapor in the air. In addition to enhancing the greenhouse effect it means that rainstorms can become more violent, as more water has to come down.
Carbon dioxide (CO2)
Carbon dioxide is the gas that almost all living creatures breathe out. Green vegetation absorbs the CO2 during daylight hours, assimilating it in the process called photosynthesis. At the same time the plants release the gas oxygen (O2 ), which animals and humans breathe in. Without oxygen we would suffocate.
The photosynthesis is the basis for all higher forms of life on Earth, as it in addition to oxygen produces glucose, a simple sugar that is the basis for more complex compounds building up the plants. Without plants, animals would no longer have food.
Increasing amounts of CO2 in the air may have a fertilizing effect on many plants if other essential requirements for photosynthesis are present. (Water, soil, minerals, right temperature etc).
Atmospheric CO2 has increased from a pre-industrial concentration of about 280 ppmv to about 400 ppmv year 2016.
(ppmv= parts per million by volume. This refers to the ratio of the number of greenhouse gas molecules to the total number of molecules of dry air. E.g.: 300 ppm = 300 molecules of GHG per million of dry air molecules.).
Atmospheric carbon dioxide (CO2 ) concentration was lingering around 280 ppmv for several thousand years until approximately 1750, the beginning of the Industrial Revolution. At that time large scale burning of fossil fuels started, while at the same time large-scale cutting of timber also took place.
NOAA: Carbon Dioxide Levels ‘Exploded’ in 2015, Highest Seen Since End of Ice Age
NASA (2016) Record annual increase of carbon dioxide observed at Mauna Loa for 2015
NASA: Graphic: The relentless rise of carbon dioxide
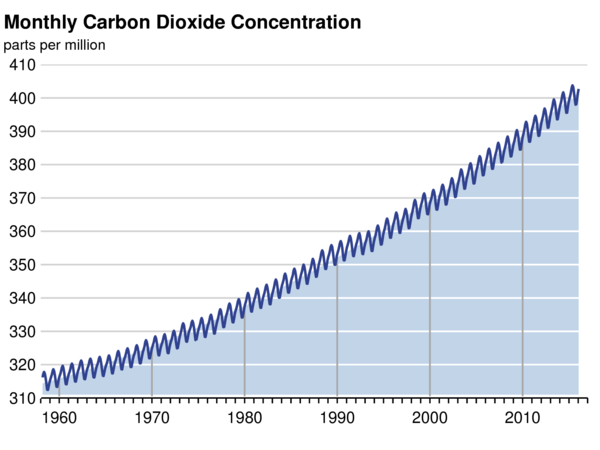
The Keeling curve – Mauna Loa observatory
The content of CO2 in the atmosphere has risen continuously since then, reaching 400 ppmv in 2015. This is an increase approaching 40%.
The present atmospheric CO2 concentration has not been exceeded during the past 420,000 years, and likely not during the past 20 million years. The rate of increase over the past century is unprecedented, at least during the past 20,000 years.
With “current energy consumption patterns the amount of carbon dioxide added to the atmosphere (as modified by absorption within natural sinks) is rising at a rate of about one half of one per cent per year” (WMO: Climate into the 21st century).
The present atmospheric CO2 increase is caused by anthropogenic emissions of CO2.
About three-quarters of these emissions are due to fossil fuel burning. Cement production and land use change is responsible for the rest of the emissions.
Methane (CH4)
This potent greenhouse gas is emitted from oil- and gas fields. Methane is released from rice paddies and from places where organic material rots without oxygen, like in marshes and big waste dumps.
Methane is also produced in the digestive system of animals, especially in ruminators like cows.
Large amounts of methane lie frozen in pockets in the Siberian permafrost areas and in frozen hydrates or clathrates on the sea floor. The Global Warming Potential (GWP) of methane is calculated to be 21 times that of CO2.
The increase of methane in the air is associated with the increase in human population and the connected increase in demand for energy, food, water, dwellings and waste dumps.
The content of methane in the atmosphere has increased about 2,5 times since 1750, from roughly 700 parts per billion by volume (ppbv) to 1803 ppbv in year 2011. This is an increase by 1060 ppb or 151%. The present concentration has not been exceeded during the past 420 000 years.
There are enormous methane deposits in ocean sediments and in permafrost areas in the Arctic. Life span of methane in the atmosphere is relatively short, only between 10-20 years, due to its reaction with oxygen.
Nitrous oxide (N2O)
This gas is emitted from artificial fertilizers in modern agriculture, from production of nitric acid and from combustion of fossil fuels. Nitrous oxide is responsible for approximately 4% of the anthropogenic greenhouse effect.
Nitrous oxide (N2O) in the atmosphere has increased from 270 ppbv in 1750 to 324 ppbv in 2011. This is an increase 20%. The increase continues.
Sources of N2O are agriculture, cattle, industry and several other natural sources. N2O is a strong greenhouse gas with a GWP of 310 times that of CO2 .The lifetime in the atmosphere is also quite long, more than 100 years.
The natural greenhouse gases are continuously being produced in nature, and are parts of finely adjusted ecological systems occuring in natural cycles. Graphic animation: Thomas Andersen, UIA
Read more:
- Greenhouse gases
- Royal Society: CO2 is already in the atmosphere naturally, so why are emissions from human activity significant?
- Explaining how the water vapor greenhouse effect works
- Sources of Greenhouse Gas Emissions (EPA)
- The Atmosphere
- Methane
- Methane emssions (EPA)
- Nitrous oxide emissions (EPA)